
2019-11-19
Evonik invests in Chinese 3D-printing start-up making medical implants
Evonik Venture Capital has invested in a 3D-printing start-up in China that makes implants for neuro and spine surgery.
- Meditool’s 3D-printed neuro and spine surgery implants mean faster patient recovery and less surgical risk
- Investment strengthens Evonik’s Smart Materials growth engine with innovative application for high-performance polymers
- Shanghai-based Meditool is Evonik Venture Capital’s first direct investment in China
Evonik Venture Capital has invested in a 3D-printing start-up in China that makes implants for neuro and spine surgery. The technology enables faster recovery and fewer post-operation checks for patients and less surgical risk for doctors. Evonik is the lead investor in a high single-digit million-euro round of fundraising for Shanghai-based Meditool.
“This is our first direct investment in China and our first direct investment after initiating our second venture capital fund this year. Meditool is a good example of how venture capital is helping Evonik secure access to disruptive technologies.” Bernhard Mohr, head of Evonik Venture Capital.
Meditool has developed its own hardware and software systems. The software can read and process images directly from commonly used magnetic resonance imaging (MRI) or computed tomography scan (CT) devices. A readily printable 3D model is generated by the software and sent to the printer. The implants are 3D printed with a high-performance polymer supplied by Evonik called Polyetheretherketone (PEEK).
“Meditool’s technology pays directly into our strategy of expanding in high-tech applications for our additive manufacturing materials. Medical applications are of particular interest and our high performance polymers have already been proven as a reliable implant material in other applications such as dental.”
Thomas Grosse-Puppendahl, head of the Evonik’s innovation growth field Additive Manufacturing.
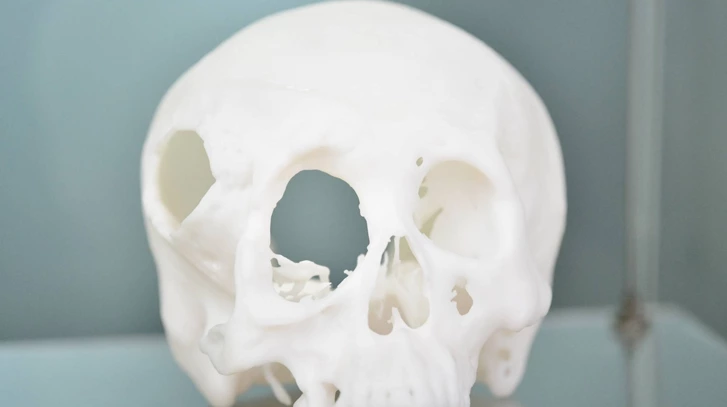

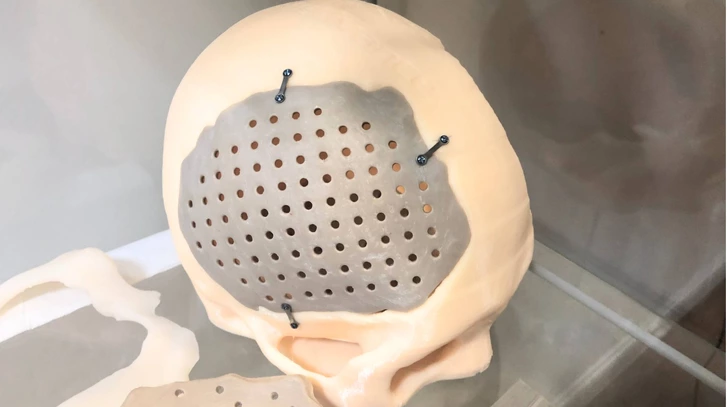
For patients and doctors, 3D-printed PEEK implants are revolutionary compared with metal, the current conventional solution for the orthopaedics implant market. 3D-printing allows customization so that, for example, a plate can be made to fit precisely to the patient’s skull. This reduces the likelihood that further operations will be required to adjust the size, shape or positioning of the implant.
PEEK is less thermally conductive than metal, meaning that patients exposed to hot and cold temperatures won’t be in danger of the implant heating up or cooling down excessively. The material is biocompatible (not harmful to living tissue) and CT and MRI examinations become possible after surgery.

“Meditool is one of the pioneers in developing 3D printed PEEK medical implants. Evonik has been our trusted partner in materials supply. The venture investment will be an extra boost to our endeavor to bring innovative solutions to patients and surgeons in China and the rest of the world.”
Ken Jin, co-founder and chief technology officer of Meditool.

“China is a key growth market for Evonik and one of the main drivers of innovation worldwide. It not only fosters leap-frog technology, it has the population and growing middle-class to drive fast-paced demand.”
Claas Klasen, President Evonik Asia North region.
The Chinese market is the world’s second largest for medical implants with expected annual growth rates of 10-15 percent.
Evonik’s venture capital arm has already invested in two funds in China and with Meditool, it now has its first direct investment. Co-investors in Meditool include ZN Ventures, Morningside Ventures and Puhua Capital.
Evonik Venture Capital plays a strategic role in Evonik’s goal to become a best-in-class specialty chemicals company, by helping secure access to disruptive technologies and innovative business models as well as supporting digital transformation.
To this end, Evonik launched its second venture capital fund with a volume of €150 million at the beginning of 2019, more than doubling the amount under management to €250 million.
Evonik markets its high-performance polymer Polyetheretherketone under the name VESTAKEEP®.
